nest - providing reliable, comprehensive answers about the health of a developing fetus
nest represents a major advance in prenatal screening, providing highly accurate answers about fetal chromosomal health—without the risks associated with invasive procedures, such as amniocentesis or chorionic villus sampling (CVS). Performed as early as 10 weeks gestation, the nest prenatal screening test demonstrates superb sensitivity and specificity for the most prevalent trisomies.
nest provides reliable, comprehensive answers about the health of a developing fetus.
nest is a simple blood test that screens for the most common chromosomal conditions that can affect a baby’s health. nest offers parents-to-be a new choice to obtain important information about the health of their developing baby, simply, accurately and in the first trimester (at 10 weeks), with little or no risk to their pregnancy.
It is performed in Australia and is supported by Genetic Counselling.
nest gives reassurance by:
- Eliminating unnecessary sample rejections. 99.9% of your patients will receive a results
- Reducing the need for redraws
- Obviating requests for paternal samples
- Providing fast report time to requesting doctor or clinic: within 5 business days after sample receipt in laboratory.
nest screening is suitable for all women at 10 weeks or greater gestation with singleton and twin pregnancies. It may be of particular value for any women who meet the following criteria:
- Advanced maternal age (≥ 35 years at delivery)
- Positive serum screen
- Ultrasound softmarker
- History suggestive of increased risk for T21, T18, or T13, or sex chromosome aneuploidy
Medical societies agree that all pregnant women should be offered prenatal screening for fetal abnormalities and that NIPT is a major advance in screening methodologies.1,2,3,4,5
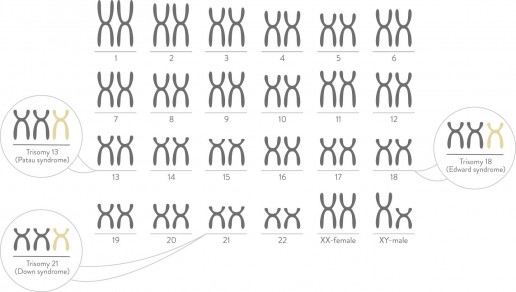
Chromosomes normally come in pairs. Most individuals usually have 23 pairs of chromosomes. nest looks for too few or too many copies of chromosomes. Missing or extra copies of chromosomes can be associated with intellectual or physical disabilities, with different levels of severity. The most commonly seen chromosomal conditions listed below can be accurately detected with nest.
Trisomy 21 Down syndrome
Carrying an extra copy of chromosome 21 causes Down syndrome. People born with Down syndrome can have health and development challenges, some level of intellectual disability and have some characteristic physical features.
Down Syndrome is the most common chromosome disorder affecting approximately one in every 660 pregnancies in Australia.
Trisomy 18 Edwards syndrome
Carrying an extra copy of chromosome 18 causes Edwards syndrome.
High rate of miscarriage is often associated with Edwards syndrome. Additional babies born with the condition have a significantly shortened life expectancy, suffer heart abnormalities, kidney malformations and developmental delays.
This is the second most common chromosome disorder affecting approximately one in every 1,100 pregnancies in Australia.
Trisomy 13 Patau syndrome
Carrying an extra copy of chromosome 13 causes Patau syndrome.
Again, with trisomy 13 high rate of miscarriage is often associated. Babies born with Patau syndrome rarely survive beyond the first year of life. They often suffer eye defects and difficulties with feeding and breathing, heart and brain problems.
Approximately one in 3,000 pregnancies in Australia are affected by Patau syndrome.
Gender Screening
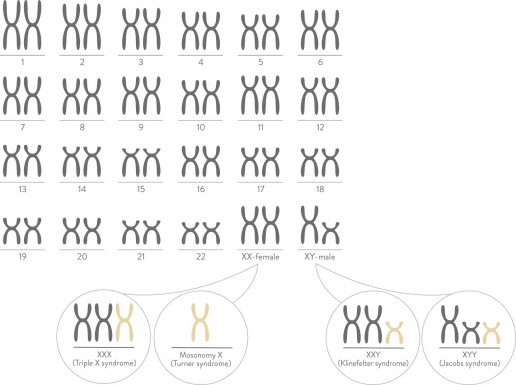
nest is also able to detect gender. Men normally have an XY pair of sex chromosomes. Women normally have an XX pair of sex chromosomes. If your doctor chooses, nest can also be used to screen for sex chromosome conditions You can elect to have gender disclosed to you or not.
- Turner syndrome – only one X chromosome in a female
- Klinefelter syndrome – an extra X chromosome in a male
- Triple X
- Jacobs syndrome- an extra Y chromosome in a male
Highly trained nest genetic counsellors are available at your request to explain the technology and how the results will be reported to your patient. They can also deliver a patients results if you require it. Having a deep understanding of this technology gives them the skills to interpret these results and assist in explaining them. If the results do come back showing a high risk result, the skills of the genetic counsellor can assist in explaining the next steps.
Screening for fetal aneuploidy in twin gestations poses unique challenges such as lower levels of DNA available for analysis from each fetus. By expanding the sensitivity and overall capability of the assay, which nest does, the test can screen twin pregnancies for T21, T18, T13 and the presence of Y chromosome (optional). The nest screening test can be used in both monozygotic and dizygotic pregnancies. Non-invasive prenatal testing (NIPT) is not recommended in the event of a vanishing or demised twin.
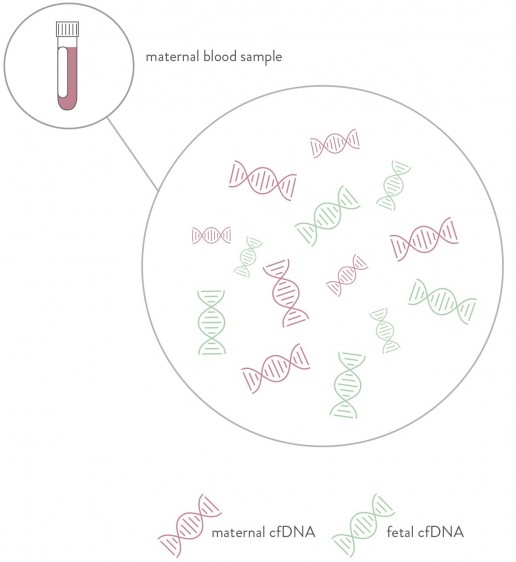
nest screening is able to detect fetal fraction. This is the amount cell-free DNA in the maternal blood that is of fetal origin. During the first trimester on average 2-10% of the DNA in the maternal blood is of fetal origin. Placental cells breakdown as part of their natural life cycle, releasing fragmented DNA particles into the maternal circulation. The inclusion of fetal fraction in NIPT screening is essential for accurate test results.
The nest prenatal screening test with our enhanced SAFeR™ algorithm increases the specific signal of aneuploid chromosomes and hence improves the overall accuracy of classifying affected samples. The test output provides unambiguous results, not a risk score, and it is not dependent on maternal age, maternal weight, gestational age (after 10 weeks) or ethnicity.
nest can also screen for the most common sex chromosome aneuploides as well as fetal sex.
Part of the nest advantage is that it provides a more stringent and optimised approach to genetic sequencing. nest leverages the power of massively parallel sequencing (MPS) across the whole genome. The industry’s deepest sequencing approach combined with a highly optimised algorithm provides a clearer, more reliable answer than other methods.
The above graph shows shallower sequencing necessitates using fetal fraction (FF) estimates as compensation for weaker sequencing power. Without FF estimates, the incidence of false negatives would be clinically unacceptable and result in higher numbers of sample rejections and delayed result time.
Utilising the power of deeper sequencing, nest gives reassurance by:
- Providing an accurate result with a low FF threshold
- Eliminating unnecessary sample rejections
- Reducing the need for redraws
- Only requires maternal blood sample
- Providing fast report time to requesting doctor or clinic: 3–5 business days after sample receipt.
nest is easy to order and needs only 1 tube of blood (just a 7mL sample). Click here to download a request form.
Our reports are available within 5 business days after sample receipt. (Time to report may vary based on the location of collection centre).
The nest screening test report is well organised and easy to read. Basic reports contain results for chromosomes 21, 18 and 13.
Test reports include one of two possible results for chromosomes 21, 18, and 13:
- Low probability
- High probability
The report will contain fetal fraction.
For singleton pregnancies, sex chromosome results are reported where requested. If there are no sex chromosome aneuploidies, then the report will indicate XX or XY status.
In the event you would like to use a nest Genetic Counsellor to assist in relaying the results to you patient please click here.
It is recommended that no irreversible clinical decisions be made based on these screening results alone. If a definitive diagnosis is desired, chorionic villus sampling or anmiocentesis should be undertaken.
With its superior technology, nest provides clinical evidence showing across-the genome analysis in a real-world population. The performance of the nest prenatal screening test was evaluated in a major scientific study that involved more than 60 leading US medical research and teaching institutions. The study findings were reviewed and published in the Journal of Obstetrics and Gynaecology in 20128. A second study, published subsequently, presented the test’s performance under regular clinical conditions and found similar results9. Monash IVF Group continues to expand the technology with its commitment to sponsor and support continued clinical studies to advance the effectiveness of NIPT (noninvasive prenatal testing). Monash IVF Group continues to innovate new solutions and is committed to sponsoring and supporting ongoing clinical studies to advance the effectiveness of NIPT (non-invasive prenatal testing).
Disclaimer
The manner in which this information is used to guide patient care is the responsibility of the health care provider, including advising for the need for genetic counselling or additional diagnostic testing. Any diagnostic testing should be interpreted in the context of all available clinical findings.
This test was developed by, and its performance characteristics were determined by, Verinata Health, Inc., a wholly-owned subsidiary of Illumina, Inc.It is registered with the Therapeutic Goods Association as an In Vitro Device.
Limitations of test
The nest prenatal test is a highly accurate, advanced screening test that is non-invasive. This test is designed to screen for chromosome aneuploidies and is validated for chromosomes 21, 18, and 13, X and Y. The test is validated for singleton and twin pregnancies with gestational age of at least 10 weeks. Genetic counselling before and after testing is recommended. These results do not eliminate the possibility that this pregnancy may be associated with other chromosomal abnormalities, birth defects, or other complications. A low probability test result does not preclude the presence of trisomy 21, trisomy 18, or trisomy 13, monosomy X, XXX, XXY, and XYY. When an aneuploidy detected result is reported in a twin pregnancy, the status of each individual fetus cannot be determined. The presence or absence of Y chromosome material can be reported in a twin pregnancy; however, the occurrence of sex chromosome aneuploidies such as MX, XXX, XXY, and XYY, cannot be evaluated in twin pregnancies. There is a small possibility that the test results might not reflect the chromosomes of the fetus, but may reflect the chromosomal changes of the placenta (confined placental mosaicism), or of the mother (chromosomal mosaicism). Results of “High Probability” are considered to be screened positive. Monash IVF Group recommends that no irreversible clinical decisions should be made based on these screening results alone. If definitive diagnosis is desired, chrionic villus sampling or amniocentesis would be necessary.
Additional Studies
Bianchi DW, Platt LD, Goldberg JD, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol . 2012;119:890–901.
Rava PP, Srinivasan A, Sehnert AJ, Bianchi DW. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem. 2014;60:243– 250.
Sehnert AJ, Rhees B, Comstock D, et al. Optimal detection of fetal chromosomal abnormalities by massively parallel DNA sequencing of cell-free fetal DNA from maternal blood. Clin Chem . 2011;57:1042–1049.
References
- ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities.Obstet Gynecol. 2007:109:217–227.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol . 2012:120:1532–1534.
- Gregg AR, Gross SJ, Best RG, et al. ACMG statement on noninvasive prenatal screening for fetal aneuploidy. Genet Med . 2013:15:395–398.
- Benn P, Borell A, Chiu R, et al. Position Statement from the Aneuploidy Screening Committee on Behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn . 2013;33:622–629.
- Devers PL, Cronister A, Ormond KE, et al. Noninvasive prenatal testing/noninvasive prenatal diagnosis: the position of the National Society of Genetic Counselors. J Genet Couns. 2013:22:291–295.
- Bhatt S, Parsa S, Snyder H, et al. Clinical Laboratory Experience with Noninvasive Prenatal Testing: Update on Clinically Relevant Metrics. ISPD 2014 poster.
- Verinata Health, Inc. (2012) Analytical Validation of the verifi prenatal test: enhanced test performance for Detecting Trisomies 21, 18, and 13 and the Option for classification of Sex Chromosome Status. Redwood City, CA.
- Bianchi DW, Platt LD, Goldberg JD, et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol . 2012;119:890–901.
- Futch T, Spinosa J, Bhatt S, et al. Initial clinical laboratory experience in noninvasive prenatal testing for fetal aneuploidy from maternal plasma DNA samples. Prenat Diagn. 2013:33:569-574.